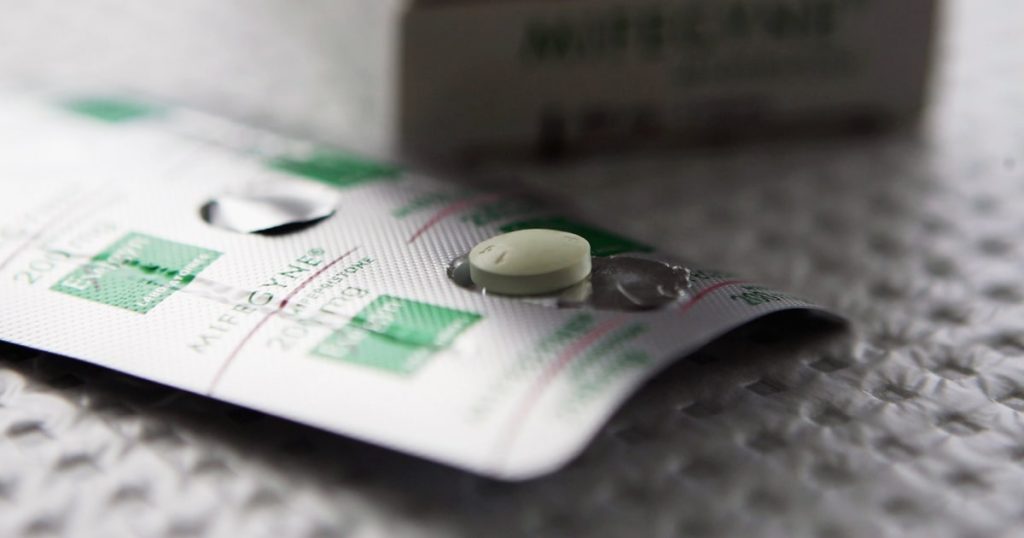
In a recent Supreme Court case, Justice Alito questioned the government’s decision to make three changes to the access of mifepristone, a medication used for medication abortion. The changes include reducing the required doctor visits from three to one, allowing patients up to 10 weeks gestation to take the drug, and permitting access to the drug by mail. Alito raised concerns about whether these changes increase the risks associated with taking mifepristone when combined. He questioned whether multiple innocuous changes could become dangerous when done together. However, Acting Solicitor General Elizabeth Prelogar argued that the studies examined by the FDA found no observable increase in serious adverse events with these changes, regardless of who is prescribing the medication.
Prelogar explained that the safety of mifepristone up to 10 weeks gestation does not rely on specific safeguards put in place by doctors versus nurse practitioners. She stated that the FDA’s exhaustive examination of the studies concluded that these changes were safe not because of additional safeguards, but because there was no observable increase in serious adverse events up to 10 weeks gestation. Alito pressed Prelogar on the potential interconnectedness and mutual reinforcement of the three changes, suggesting that the combination of these changes could pose a greater risk than each change individually. However, Prelogar maintained that the evidence presented in the record did not support this claim, pointing to the FDA’s findings that the changes did not result in a higher rate of serious adverse events.
The discussion on the safety of mifepristone highlights the ongoing legal battles surrounding access to abortion medications in the United States. The debate over telehealth access, mail-in prescriptions, and restrictions on medication abortion has been a contentious issue between proponents of reproductive rights and anti-abortion advocates. The Supreme Court’s decision on this case could have significant implications for the access and regulation of medication abortion, as well as broader implications for reproductive health rights in the country. The arguments presented by Alito and Prelogar reflect the larger debate over the regulation of medication abortion and the balancing of safety concerns with access to reproductive healthcare.
Alito’s questions during the hearing underscore the complexity of the issues surrounding medication abortion and highlight the potential for differing interpretations of the available evidence. The Supreme Court’s decision will ultimately determine whether the government’s changes to the access of mifepristone are justified from a safety perspective or pose unnecessary risks to patients. With the conservative majority on the Court, the decision could have far-reaching implications for the future of reproductive healthcare and abortion access in the United States. The outcome of this case will likely shape the legal landscape surrounding medication abortion and impact the ability of individuals to access this form of reproductive healthcare in the future.
As the Supreme Court continues to review cases related to reproductive rights, the debate over medication abortion and access to healthcare services remains a contentious and polarizing issue in the United States. The legal battle over the changes to mifepristone access reflects broader disagreements over reproductive rights, women’s healthcare, and the government’s role in regulating medical procedures. The arguments presented by both sides in this case highlight the complex considerations at play and the potential implications of the Supreme Court’s decision on the future of abortion access in the country. Ultimately, the Court’s ruling in this case will have a significant impact on the landscape of reproductive rights and healthcare in the United States.